
-
 Indigenous Brazilians protest Amazon river dredging for grain exports
Indigenous Brazilians protest Amazon river dredging for grain exports
-
Google's annual revenue tops $400 bn for first time, AI investments rise

-
 Last US-Russia nuclear treaty ends in 'grave moment' for world
Last US-Russia nuclear treaty ends in 'grave moment' for world
-
Man City brush aside Newcastle to reach League Cup final

-
 Guardiola wants permission for Guehi to play in League Cup final
Guardiola wants permission for Guehi to play in League Cup final
-
Boxer Khelif reveals 'hormone treatments' before Paris Olympics

-
 'Bad Boy,' 'Little Pablo' and Mordisco: the men on a US-Colombia hitlist
'Bad Boy,' 'Little Pablo' and Mordisco: the men on a US-Colombia hitlist
-
BHP damages trial over Brazil mine disaster to open in 2027

-
 Dallas deals Davis to Wizards in blockbuster NBA trade: report
Dallas deals Davis to Wizards in blockbuster NBA trade: report
-
Lens cruise into French Cup quarters, Endrick sends Lyon through

-
 No.1 Scheffler excited for Koepka return from LIV Golf
No.1 Scheffler excited for Koepka return from LIV Golf
-
Curling quietly kicks off sports programme at 2026 Winter Olympics

-
 Undav pokes Stuttgart past Kiel into German Cup semis
Undav pokes Stuttgart past Kiel into German Cup semis
-
Germany goalkeeper Ter Stegen to undergo surgery

-
 Bezos-led Washington Post announces 'painful' job cuts
Bezos-led Washington Post announces 'painful' job cuts
-
Iran says US talks are on, as Trump warns supreme leader

-
 Gaza health officials say strikes kill 24 after Israel says officer wounded
Gaza health officials say strikes kill 24 after Israel says officer wounded
-
Empress's crown dropped in Louvre heist to be fully restored: museum

-
 UK PM says Mandelson 'lied' about Epstein relations
UK PM says Mandelson 'lied' about Epstein relations
-
Shai to miss NBA All-Star Game with abdominal strain

-
 Trump suggests 'softer touch' needed on immigration
Trump suggests 'softer touch' needed on immigration
-
From 'flop' to Super Bowl favorite: Sam Darnold's second act

-
 Man sentenced to life in prison for plotting to kill Trump in 2024
Man sentenced to life in prison for plotting to kill Trump in 2024
-
Native Americans on high alert over Minneapolis crackdown

-
 Dallas deals Davis to Wizards in blockbuster NBA deal: report
Dallas deals Davis to Wizards in blockbuster NBA deal: report
-
Panama hits back after China warns of 'heavy price' in ports row

-
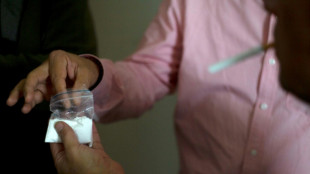 Strike kills guerrillas as US, Colombia agree to target narco bosses
Strike kills guerrillas as US, Colombia agree to target narco bosses
-
Wildfire smoke kills more than 24,000 Americans a year: study

-
 Telegram founder slams Spain PM over under-16s social media ban
Telegram founder slams Spain PM over under-16s social media ban
-
Curling kicks off sports programme at 2026 Winter Olympics

-
 Preventative cholera vaccination resumes as global supply swells: WHO
Preventative cholera vaccination resumes as global supply swells: WHO
-
Wales' Macleod ready for 'physical battle' against England in Six Nations

-
 Xi calls for 'mutual respect' with Trump, hails ties with Putin
Xi calls for 'mutual respect' with Trump, hails ties with Putin
-
'All-time great': Maye's ambitions go beyond record Super Bowl bid

-
 Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
-
US seeks minerals trade zone in rare Trump move with allies

-
 Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
-
Brazil mine disaster victims in London to 'demand what is owed'

-
 AI-fuelled tech stock selloff rolls on
AI-fuelled tech stock selloff rolls on
-
White says time at Toulon has made him a better Scotland player

-
 Washington Post announces 'painful' job cuts
Washington Post announces 'painful' job cuts
-
All lights are go for Jalibert, says France's Dupont

-
 Artist rubs out Meloni church fresco after controversy
Artist rubs out Meloni church fresco after controversy
-
Palestinians in Egypt torn on return to a Gaza with 'no future'

-
 US removing 700 immigration officers from Minnesota
US removing 700 immigration officers from Minnesota
-
Who is behind the killing of late ruler Gaddafi's son, and why now?

-
 Coach Thioune tasked with saving battling Bremen
Coach Thioune tasked with saving battling Bremen
-
Russia vows to act 'responsibly' once nuclear pact with US ends

-
 Son of Norway's crown princess admits excesses but denies rape
Son of Norway's crown princess admits excesses but denies rape
-
Vowles dismisses Williams 2026 title hopes as 'not realistic'


US congressional report slams FDA approval of Alzheimer's drug
The US Food and Drug Administration's approval process for a controversial drug used to treat Alzheimer's was "rife with irregularities," a congressional report said Thursday.
An 18-month investigation into the FDA's green-lighting of the drug, Aduhelm, also criticized its manufacturer, biotechnology company Biogen.
The Cambridge, Massachusetts-based Biogen set an "unjustifiably high price" for Aduhelm of $56,000 a year to "make history" with the first drug approved in decades to treat Alzheimer's, the report said.
Aduhelm received "accelerated approval" from the FDA in June despite the fact that an independent panel advising the US drug regulator had found insufficient evidence of its benefit and some experts had raised concerns about inconsistency in the drug's clinical data.
At least three of the 11-member independent committee that voted unanimously against recommending the drug to the FDA subsequently resigned.
According to the congressional investigators, the FDA "considered Aduhelm under the traditional approval pathway used for most drugs for nine months, before abruptly changing course and granting approval under the accelerated approval pathway after a three-week review period."
They found that FDA interactions with Biogen were "atypical" and included a failure to properly document contacts between agency staff and the drug maker.
The FDA and Biogen had also "inappropriately collaborated" on a joint briefing document for a key advisory committee.
"FDA's approval process was rife with irregularities," the report said.
As for Biogen, the report said the company "viewed Aduhelm as an unprecedented financial opportunity -- estimating a potential peak revenue of $18 billion per year."
It quoted a September 2020 presentation to the Biogen board as saying: "Our ambition is to make history" and to "establish Aduhelm as one of the top pharmaceutical launches of all time."
- 'Wake-up call' -
Carolyn Maloney, chairwoman of the House Oversight and Reform Committee, said she hoped the report's findings "are a wake-up call for FDA to reform its practices."
Frank Pallone, chairman of the House Energy and Commerce Committee, said the report "documents the atypical FDA review process and corporate greed that preceded FDA's controversial decision to grant accelerated approval to Aduhelm."
"While we all support the search for new cures and treatments to address devastating diseases like Alzheimer's, we must ensure that expediency does not take precedence over protocols," Pallone said. "Patient safety and drug efficacy must remain at the core of our nation's pharmaceutical regulatory review process."
In a statement, the FDA said it "remains committed to the integrity of our drug approval process, which includes ensuring that safe and effective new treatment options are available to the millions of people with Alzheimer's disease."
Biogen said it "stands by the integrity of the actions we have taken."
"Biogen has been committed to researching and developing treatments for Alzheimer's disease for more than a decade," the company said.
"We have been focused relentlessly on innovation to address this global health challenge, and have adapted to both successes and setbacks."
A.Aguiar--PC


