-
 Selection process for future Olympic hosts set for reform
Selection process for future Olympic hosts set for reform
-
Serbian minister on trial over Trump-linked hotel plan

-
 UK PM says Mandelson 'lied', regrets appointing him US envoy
UK PM says Mandelson 'lied', regrets appointing him US envoy
-
Cochran-Siegle tops first Olympic downhill training

-
 Gaza health officials say strikes kill 21 after Israel says shots wounded officer
Gaza health officials say strikes kill 21 after Israel says shots wounded officer
-
Injured Vonn's Olympic bid is 'inspirational', ski stars say

-
 Albania arrests 20 for toxic waste trafficking
Albania arrests 20 for toxic waste trafficking
-
US-Africa trade deal renewal only 'temporary breather'

-
 Mir sets pace on Sepang day two, Yamaha absent
Mir sets pace on Sepang day two, Yamaha absent
-
Xi, Putin hail 'stabilising' China-Russia alliance

-
 GSK boosted by specialty drugs, end to Zantac fallout
GSK boosted by specialty drugs, end to Zantac fallout
-
UK's ex-prince leaves Windsor home amid Epstein storm: reports

-
 Sky is the limit for Ireland fly-half Prendergast, says captain Doris
Sky is the limit for Ireland fly-half Prendergast, says captain Doris
-
Feyi-Waboso reminds England great Robinson of himself

-
 Starmer faces MPs as pressure grows over Mandelson scandal
Starmer faces MPs as pressure grows over Mandelson scandal
-
HRW urges pushback against 'aggressive superpowers'

-
 Russia demands Ukraine give in as UAE talks open
Russia demands Ukraine give in as UAE talks open
-
Gaza civil defence says 17 killed in strikes after Israel says shots wounded officer

-
 France's Kante joins Fenerbahce after Erdogan 'support'
France's Kante joins Fenerbahce after Erdogan 'support'
-
CK Hutchison launches arbitration over Panama Canal port ruling

-
 Stocks mostly rise as traders ignore AI-fuelled sell-off on Wall St
Stocks mostly rise as traders ignore AI-fuelled sell-off on Wall St
-
Acclaimed Iraqi film explores Saddam Hussein's absurd birthday rituals

-
 On rare earth supply, Trump for once seeks allies
On rare earth supply, Trump for once seeks allies
-
Ukrainian chasing sumo greatness after meteoric rise

-
 Draper to make long-awaited return in Davis Cup qualifier
Draper to make long-awaited return in Davis Cup qualifier
-
Can Ilia Malinin fulfil his promise at the Winter Olympics?

-
 CK Hutchison begins arbitration against Panama over annulled canal contract
CK Hutchison begins arbitration against Panama over annulled canal contract
-
UNESCO recognition inspires hope in Afghan artist's city

-
 Ukraine, Russia, US negotiators gather in Abu Dhabi for war talks
Ukraine, Russia, US negotiators gather in Abu Dhabi for war talks
-
WTO must 'reform or die': talks facilitator

-
 Doctors hope UK archive can solve under-50s bowel cancer mystery
Doctors hope UK archive can solve under-50s bowel cancer mystery
-
Stocks swing following latest AI-fuelled sell-off on Wall St

-
 Demanding Dupont set to fire France in Ireland opener
Demanding Dupont set to fire France in Ireland opener
-
Britain's ex-prince Andrew leaves Windsor home: BBC

-
 Coach plots first South Africa World Cup win after Test triumph
Coach plots first South Africa World Cup win after Test triumph
-
Spin-heavy Pakistan hit form, but India boycott risks early T20 exit

-
 Japan eyes Premier League parity by aligning calendar with Europe
Japan eyes Premier League parity by aligning calendar with Europe
-
Whack-a-mole: US academic fights to purge his AI deepfakes

-
 Love in a time of war for journalist and activist in new documentary
Love in a time of war for journalist and activist in new documentary
-
'Unprecedented mass killing': NGOs battle to quantify Iran crackdown scale

-
 Seahawks kid Cooper Kupp seeks new Super Bowl memories
Seahawks kid Cooper Kupp seeks new Super Bowl memories
-
Thousands of Venezuelans march to demand Maduro's release

-
 AI, manipulated images falsely link some US politicians with Epstein
AI, manipulated images falsely link some US politicians with Epstein
-
Move on, says Trump as Epstein files trigger probe into British politician

-
 Axon Neuroscience's Immunotherapy Selected for a Landmark Combination-Therapy Alzheimer’s Clinical Trial in US, Supported by a USD 151 Million Grant
Axon Neuroscience's Immunotherapy Selected for a Landmark Combination-Therapy Alzheimer’s Clinical Trial in US, Supported by a USD 151 Million Grant
-
CHAR Technologies Licenses High-Temperature Pyrolysis Technology to GazoTech SAS for Entry Into European Markets

-
 Arteta backs Arsenal to build on 'magical' place in League Cup final
Arteta backs Arsenal to build on 'magical' place in League Cup final
-
Evil Empire to underdogs: Patriots eye 7th Super Bowl

-
 UBS grilled on Capitol Hill over Nazi-era probe
UBS grilled on Capitol Hill over Nazi-era probe
-
Guardiola 'hurt' by suffering caused in global conflicts


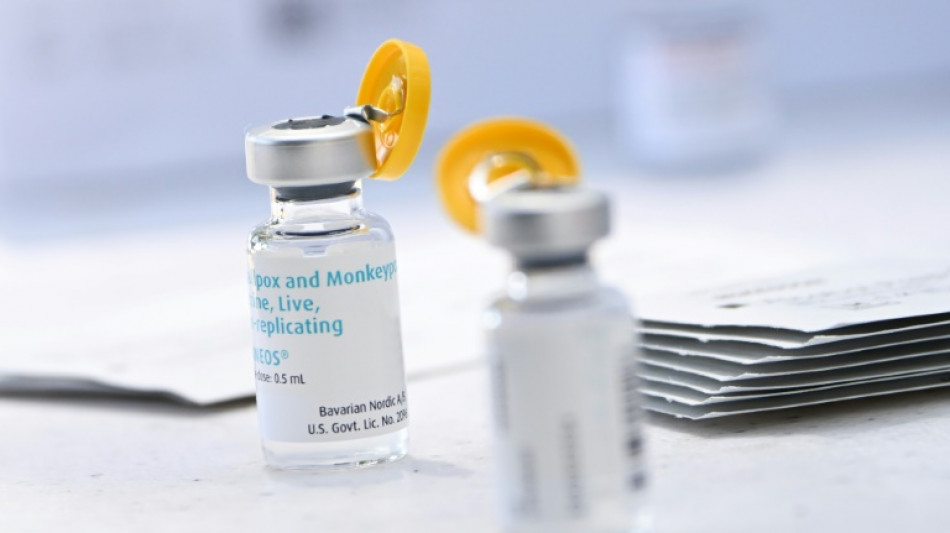
New US strategy to make monkeypox vaccine go further
US health authorities on Tuesday authorized a new procedure for injecting the monkeypox vaccine that should make it possible to inoculate more people with the same amount of the drug, at a time when doses are running short in the country.
The Food and Drug Administration also authorized giving the vaccine to people under the age of 18 who are considered to be at high risk of infection.
For those over 18, health workers will now be able to administer the vaccine differently, via an intradermal injection -- that is, between the upper layers of the skin -- and not with a deeper, subcutaneous injection.
The new method will "increase the total number of doses available for use by up to five-fold," the FDA said in a statement.
Two injections, four weeks apart, will still be necessary.
The FDA said it was drawing on data from a 2015 clinical trial that showed a similar immune response in people given a subcutaneous injection compared to those given a fifth of the dose via an intradermal shot.
At present, some 620,000 doses of the vaccine -- manufactured by Bavarian Nordic, and marketed under the name Jynneos in the United States -- have been distributed across the country.
Another 440,000 additional doses are still to be distributed, which could allow up to 2.2 million injections under the new strategy.
The government has also ordered an additional five million doses, which will start arriving from September and run through 2023, affording the potential to administer 25 million doses.
The decisions came after the FDA issued an emergency use authorization for the vaccine, a move that itself followed the declaration of a public health emergency last week.
For the authorization in minors, the FDA said it had reviewed safety data for the vaccine, as well as data for another vaccine given in children against smallpox.
"We feel very comfortable with the safety of the approach," said Peter Marks of the FDA at a press conference, noting a recent increase in the number of children who have potentially been exposed to infected people.
The United States has registered nearly 9,000 cases of monkeypox, a fifth of them in New York state. The vast majority of cases involve men who have had sex with men.
X.M.Francisco--PC


