-
 Rijksmuseum puts the spotlight on Roman poet's epic
Rijksmuseum puts the spotlight on Roman poet's epic
-
Trump fuels EU push to cut cord with US tech

-
 Fearless talent: Five young players to watch at the T20 World Cup
Fearless talent: Five young players to watch at the T20 World Cup
-
India favourites as T20 World Cup to begin after chaotic build-up

-
 Voter swings raise midterm alarm bells for Trump's Republicans
Voter swings raise midterm alarm bells for Trump's Republicans
-
Australia dodges call for arrest of visiting Israel president

-
 Countries using internet blackouts to boost censorship: Proton
Countries using internet blackouts to boost censorship: Proton
-
Top US news anchor pleads with kidnappers for mom's life

-
 Thailand's pilot PM on course to keep top job
Thailand's pilot PM on course to keep top job
-
The coming end of ISS, symbol of an era of global cooperation

-
 New crew set to launch for ISS after medical evacuation
New crew set to launch for ISS after medical evacuation
-
Family affair: Thailand waning dynasty still election kingmaker

-
 Japan's first woman PM tipped for thumping election win
Japan's first woman PM tipped for thumping election win
-
Stocks in retreat as traders reconsider tech investment

-
 LA officials call for Olympic chief to resign over Epstein file emails
LA officials call for Olympic chief to resign over Epstein file emails
-
Ukraine, Russia, US to start second day of war talks

-
 Fiji football legend returns home to captain first pro club
Fiji football legend returns home to captain first pro club
-
Trump attacks US electoral system with call to 'nationalize' voting

-
 Barry Manilow cancels Las Vegas shows but 'doing great' post-surgery
Barry Manilow cancels Las Vegas shows but 'doing great' post-surgery
-
US households become increasingly strained in diverging economy

-
 Four dead men: the cold case that engulfed a Colombian cycling star
Four dead men: the cold case that engulfed a Colombian cycling star
-
Super Bowl stars stake claims for Olympic flag football

-
 On a roll, Brazilian cinema seizes its moment
On a roll, Brazilian cinema seizes its moment
-
Rising euro, falling inflation in focus at ECB meeting

-
 AI to track icebergs adrift at sea in boon for science
AI to track icebergs adrift at sea in boon for science
-
Indigenous Brazilians protest Amazon river dredging for grain exports

-
 Google's annual revenue tops $400 bn for first time, AI investments rise
Google's annual revenue tops $400 bn for first time, AI investments rise
-
Last US-Russia nuclear treaty ends in 'grave moment' for world

-
 Man City brush aside Newcastle to reach League Cup final
Man City brush aside Newcastle to reach League Cup final
-
Guardiola wants permission for Guehi to play in League Cup final

-
 Boxer Khelif reveals 'hormone treatments' before Paris Olympics
Boxer Khelif reveals 'hormone treatments' before Paris Olympics
-
'Bad Boy,' 'Little Pablo' and Mordisco: the men on a US-Colombia hitlist

-
 BHP damages trial over Brazil mine disaster to open in 2027
BHP damages trial over Brazil mine disaster to open in 2027
-
Dallas deals Davis to Wizards in blockbuster NBA trade: report

-
 Lens cruise into French Cup quarters, Endrick sends Lyon through
Lens cruise into French Cup quarters, Endrick sends Lyon through
-
No.1 Scheffler excited for Koepka return from LIV Golf

-
 Curling quietly kicks off sports programme at 2026 Winter Olympics
Curling quietly kicks off sports programme at 2026 Winter Olympics
-
Undav pokes Stuttgart past Kiel into German Cup semis

-
 Germany goalkeeper Ter Stegen to undergo surgery
Germany goalkeeper Ter Stegen to undergo surgery
-
Bezos-led Washington Post announces 'painful' job cuts

-
 Iran says US talks are on, as Trump warns supreme leader
Iran says US talks are on, as Trump warns supreme leader
-
Gaza health officials say strikes kill 24 after Israel says officer wounded

-
 Empress's crown dropped in Louvre heist to be fully restored: museum
Empress's crown dropped in Louvre heist to be fully restored: museum
-
UK PM says Mandelson 'lied' about Epstein relations

-
 Shai to miss NBA All-Star Game with abdominal strain
Shai to miss NBA All-Star Game with abdominal strain
-
Trump suggests 'softer touch' needed on immigration

-
 From 'flop' to Super Bowl favorite: Sam Darnold's second act
From 'flop' to Super Bowl favorite: Sam Darnold's second act
-
Man sentenced to life in prison for plotting to kill Trump in 2024

-
 Native Americans on high alert over Minneapolis crackdown
Native Americans on high alert over Minneapolis crackdown
-
Dallas deals Davis to Wizards in blockbuster NBA deal: report


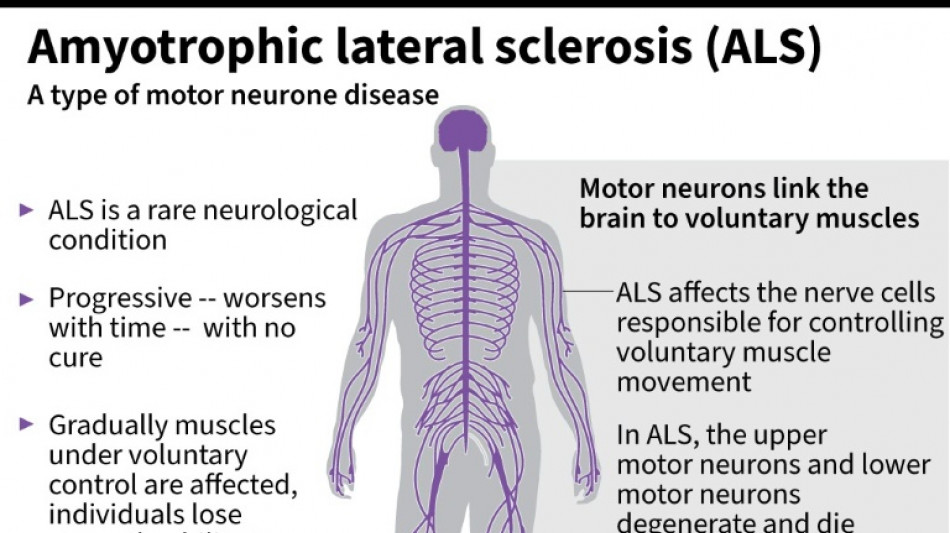
Every month counts: European ALS patients want new drugs
Olivier Goy is running out of time.
The French entrepreneur was diagnosed in 2020 with amyotrophic lateral sclerosis (ALS) -- the incurable neurodegenerative disease that normally claims the lives of patients within three to five years.
There are new treatments that have given patients hope of being able to extend their lives by an invaluable few months, but the approval process in Europe is taking time, infuriating desperate patients.
"When you are certain to die soon, patients and some doctors are ready to take some risks," Goy told AFP.
In response to the lack of new treatments in his native France, the founder of the fintech start-up October spends 3,000 euros ($3,180) every month to buy the ingredients to make his own drugs.
ALS, also known as Lou Gehrig's disease, attacks the motor nerve cells in the brain and spinal cord, progressively paralysing muscles until patients cannot walk, eat, speak or breathe.
Around one in 10,000 people have the disease in the EU, according to the European Medicines Agency.
The drug Riluzole, which has been available in Europe and the UK since the 1990s, is capable of prolonging the lives of patients by around three months.
But otherwise, no new treatment has been approved in Europe for more than two decades.
- 'First hope in 20 years' -
A new treatment called AMX0035 was given the green light in the United States and Canada last year.
"It is the first hope we have had in 20 years: the first drug which is aimed at everyone and which had results" suggesting up to six months in added life expectancy, said Sabine Turgeman, head of the French Association for Research into ALS.
But the extent of the benefits of AMX0035 remains unclear. The US Food and Drug Administration approved the drug, sold under the name Relyvrio, based on the results of a single Phase 2 trial that involved just 137 participants.
The drug's developer, Amylyx Pharmaceuticals, is conducting larger, more comprehensive trials, with results expected in 2024.
Amylyx said earlier this month that the European Union's drug watchdog EMA is reviewing its submission for approval and it expects a decision in the first half of this year.
But for those with the disease, every delay represents a significant amount of the time they have left.
"It's not going fast enough," Turgeman said. "This disease is not on bureaucratic time".
For European patients who cannot afford to import their own ingredients like Goy, the only way to get access to new treatments is to join a clinical trial.
But such trials have very specific criteria for selection -- and even if a patient gets in, there is a chance they will be in the group given a placebo.
- 'Totally abandoned' -
Given how swiftly the disease progresses, patients and families are pressing for more options.
"We feel totally abandoned," said Sophie Garofalo, whose brother was diagnosed with ALS five years ago.
His family tried to enter him into clinical trials, "but either he does not meet the criteria, or the trials have already started," she said.
"He is ready to take anything, try everything".
French pharmaceutical company AB Science is developing another potential treatment using the drug masitinib, which initial results suggest could add months to the lives of patients.
The firm's CEO Alain Moussy said that because "time is very limited" for ALS patients, there should be more flexibility in the approval system.
"What degree of risk should be taken? That's for the health agencies to answer -- but they can guided by policymakers and patients," he said.
F.Carias--PC



