-
 CK Hutchison launches arbitration over Panama Canal port ruling
CK Hutchison launches arbitration over Panama Canal port ruling
-
Stocks mostly rise as traders ignore AI-fuelled sell-off on Wall St

-
 Acclaimed Iraqi film explores Saddam Hussein's absurd birthday rituals
Acclaimed Iraqi film explores Saddam Hussein's absurd birthday rituals
-
On rare earth supply, Trump for once seeks allies

-
 Ukrainian chasing sumo greatness after meteoric rise
Ukrainian chasing sumo greatness after meteoric rise
-
Draper to make long-awaited return in Davis Cup qualifier

-
 Can Ilia Malinin fulfil his promise at the Winter Olympics?
Can Ilia Malinin fulfil his promise at the Winter Olympics?
-
CK Hutchison begins arbitration against Panama over annulled canal contract

-
 UNESCO recognition inspires hope in Afghan artist's city
UNESCO recognition inspires hope in Afghan artist's city
-
Ukraine, Russia, US negotiators gather in Abu Dhabi for war talks

-
 WTO must 'reform or die': talks facilitator
WTO must 'reform or die': talks facilitator
-
Doctors hope UK archive can solve under-50s bowel cancer mystery

-
 Stocks swing following latest AI-fuelled sell-off on Wall St
Stocks swing following latest AI-fuelled sell-off on Wall St
-
Demanding Dupont set to fire France in Ireland opener

-
 Britain's ex-prince Andrew leaves Windsor home: BBC
Britain's ex-prince Andrew leaves Windsor home: BBC
-
Coach plots first South Africa World Cup win after Test triumph

-
 Spin-heavy Pakistan hit form, but India boycott risks early T20 exit
Spin-heavy Pakistan hit form, but India boycott risks early T20 exit
-
Japan eyes Premier League parity by aligning calendar with Europe

-
 Whack-a-mole: US academic fights to purge his AI deepfakes
Whack-a-mole: US academic fights to purge his AI deepfakes
-
Love in a time of war for journalist and activist in new documentary

-
 'Unprecedented mass killing': NGOs battle to quantify Iran crackdown scale
'Unprecedented mass killing': NGOs battle to quantify Iran crackdown scale
-
Seahawks kid Cooper Kupp seeks new Super Bowl memories

-
 Thousands of Venezuelans march to demand Maduro's release
Thousands of Venezuelans march to demand Maduro's release
-
AI, manipulated images falsely link some US politicians with Epstein

-
 Move on, says Trump as Epstein files trigger probe into British politician
Move on, says Trump as Epstein files trigger probe into British politician
-
Arteta backs Arsenal to build on 'magical' place in League Cup final

-
 Evil Empire to underdogs: Patriots eye 7th Super Bowl
Evil Empire to underdogs: Patriots eye 7th Super Bowl
-
UBS grilled on Capitol Hill over Nazi-era probe

-
 Guardiola 'hurt' by suffering caused in global conflicts
Guardiola 'hurt' by suffering caused in global conflicts
-
Marseille do their work early to beat Rennes in French Cup

-
 Trump signs spending bill ending US government shutdown
Trump signs spending bill ending US government shutdown
-
Arsenal sink Chelsea to reach League Cup final

-
 Leverkusen sink St Pauli to book spot in German Cup semis
Leverkusen sink St Pauli to book spot in German Cup semis
-
'We just need something positive' - Monks' peace walk across US draws large crowds

-
 Milan close gap on Inter with 3-0 win over Bologna
Milan close gap on Inter with 3-0 win over Bologna
-
No US immigration agents at Super Bowl: security chief

-
 NASA Moon mission launch delayed to March after test
NASA Moon mission launch delayed to March after test
-
Spain to seek social media ban for under-16s

-
 LIV Golf events to receive world ranking points: official
LIV Golf events to receive world ranking points: official
-
US House passes spending bill ending government shutdown

-
 US jet downs Iran drone but talks still on course
US jet downs Iran drone but talks still on course
-
UK police launching criminal probe into ex-envoy Mandelson

-
 US-Iran talks 'still scheduled' after drone shot down: White House
US-Iran talks 'still scheduled' after drone shot down: White House
-
Chomsky sympathized with Epstein over 'horrible' press treatment

-
 French prosecutors stick to demand for five-year ban for Le Pen
French prosecutors stick to demand for five-year ban for Le Pen
-
Russia's economic growth slowed to 1% in 2025: Putin

-
 Bethell spins England to 3-0 sweep over Sri Lanka in World Cup warm-up
Bethell spins England to 3-0 sweep over Sri Lanka in World Cup warm-up
-
Nagelsmann backs Ter Stegen for World Cup despite 'cruel' injury

-
 Homage or propaganda? Carnival parade stars Brazil's Lula
Homage or propaganda? Carnival parade stars Brazil's Lula
-
EU must be 'less naive' in COP climate talks: French ministry


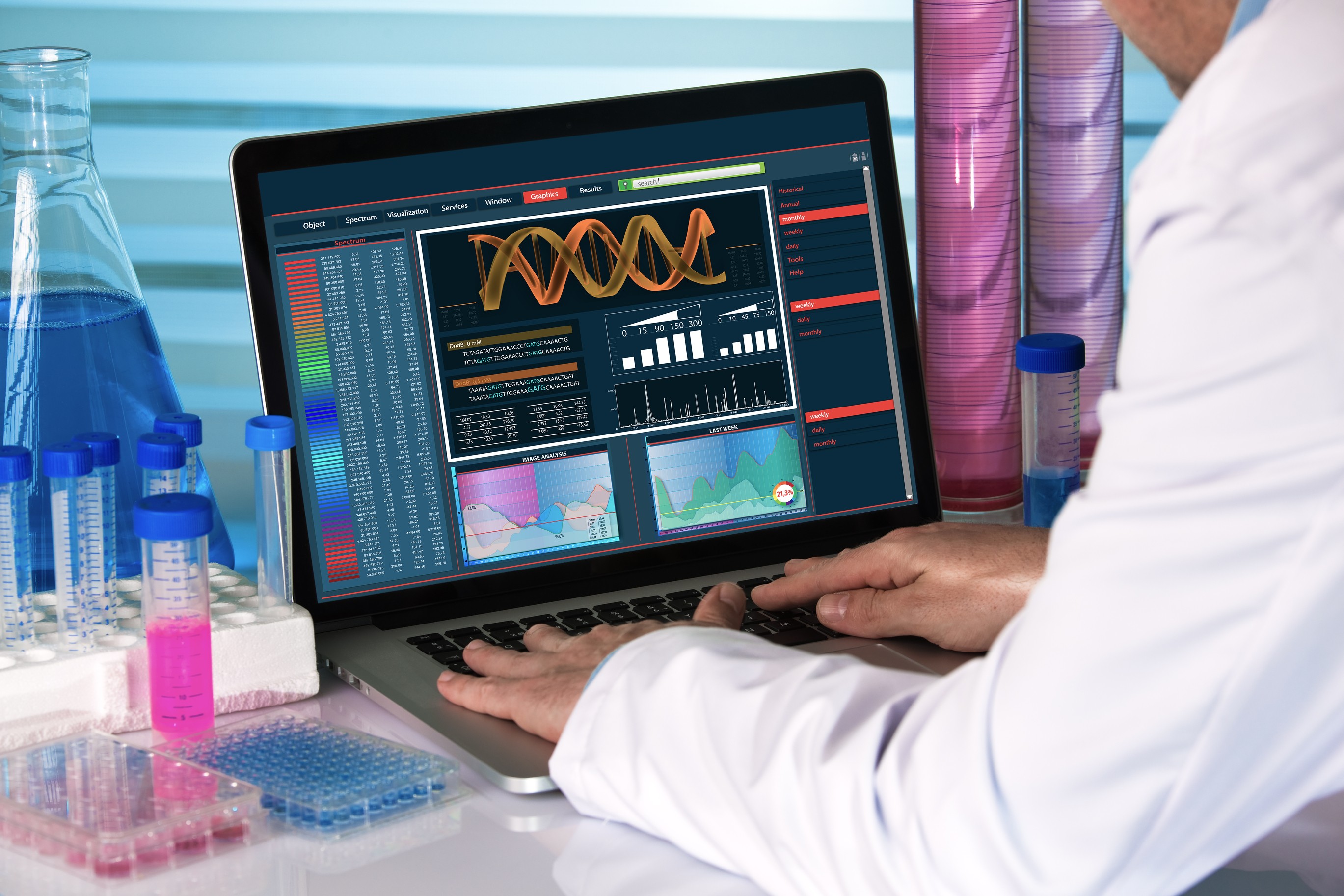
Northway Biotech Launches Full-Service Viral Clearance Studies, Delivering Results Faster Than Industry Standards
With six newly established, identical BSL-2 laboratories now operational, biologics CDMO Northway Biotech can conduct VCS programs for up to six clients simultaneously, significantly alleviating current market bottlenecks
VILNIUS, LT / ACCESS Newswire / May 5, 2025 / Northway Biotech, a biopharmaceutical contract development and manufacturing organization (CDMO), today announced the expansion of its protein-based and gene therapy service offerings with the addition of Viral Clearance Studies (VCS) capabilities. This strategic growth follows the opening of Northway Biotech's new Gene Therapy Center with dedicated cGMP facilities for virus-related projects.
With six newly established, identical BSL-2 laboratories now operational, Northway Biotech can conduct VCS programs for up to six clients simultaneously, significantly alleviating current market bottlenecks. Additionally, the company has expanded its capabilities to perform GMP-compliant manufacturing and testing under BSL-3 conditions, further strengthening its service offering across gene therapy and broader biologics development.
Viral Clearance Studies are now offered both as part of Northway Biotech's integrated CDMO programs and as a standalone service. This flexibility allows external clients to access VCS expertise independently, without requiring a manufacturing agreement.
Accelerated Delivery Timelines - Over One Month Faster Than Industry
Leveraging expanded infrastructure and integrated analytical capabilities, Northway Biotech is positioned to deliver Viral Clearance Studies substantially faster than the current industry standard. Comprehensive studies, assessing viral removal and inactivation, can now be completed with final regulatory-compliant reporting in under 10 weeks from initiation of project design when two model viruses are employed, and within 12 weeks when four model viruses are used.
"Our expansion into Viral Clearance Studies is a natural extension of our CDMO services, enabling us to manage these critical studies in-house and significantly reduce project timelines for our clients," said Prof. Vladas Algirdas Bumelis, CEO of Northway Biotech. "By investing in state-of-the-art BSL-2 and BSL-3 facilities, expanding technical capabilities, and further strengthening our scientific teams, we are uniquely positioned to deliver high-quality VCS data faster - a key advantage for clients advancing through clinical development and regulatory approval."
For more information on Northway Biotech's Viral Clearance Study processes, service offerings, and delivery timelines, please complete the contact form to connect with the Northway Biotech team.
About Northway Biotech - https://www.northwaybiotech.com
Northway Biotech is a leading contract development and manufacturing organization (CDMO) supporting customers worldwide. Its highly experienced and professional team executes projects at every stage, from cell line construction and process development to cGMP manufacturing of biopharmaceutical products. The company's extensive expertise and vertically integrated service offering enables rapid execution of multiple projects from its state-of-the-art GMP facilities while ensuring full process and product compliance at all stages of research, development, and commercial manufacturing. Northway Biotech is a privately owned company founded in 2004 and operates locations in Vilnius, Lithuania; London, United Kingdom; and Waltham, MA, USA.
Media & Business Contact:
Prof. Vladas Algirdas Bumelis
CEO and Chairman of the Board
Northway Biotech
[email protected]
Contact Information
Vladas Bumelis
CEO and Chairman of the Board
[email protected]
SOURCE: Northway Biotech
View the original press release on ACCESS Newswire
A.Silveira--PC

