-
 Colombia's Petro meets Trump after months of tensions
Colombia's Petro meets Trump after months of tensions
-
Air India inspects Boeing 787 fuel switches after grounding

-
 US envoy evokes transition to 'democratic' Venezuela
US envoy evokes transition to 'democratic' Venezuela
-
Syria govt forces enter Qamishli under agreement with Kurds

-
 WHO wants $1 bn for world's worst health crises in 2026
WHO wants $1 bn for world's worst health crises in 2026
-
France summons Musk, raids X offices as deepfake backlash grows

-
 Four out of every 10 cancer cases are preventable: WHO
Four out of every 10 cancer cases are preventable: WHO
-
Sacked UK envoy Mandelson quits parliament over Epstein ties

-
 US House to vote Tuesday to end partial government shutdown
US House to vote Tuesday to end partial government shutdown
-
Eswatini minister slammed for reported threat to expel LGBTQ pupils

-
 Pfizer shares drop on quarterly loss
Pfizer shares drop on quarterly loss
-
Norway's Kilde withdraws from Winter Olympics

-
 Vonn says 'confident' can compete at Olympics despite ruptured ACL
Vonn says 'confident' can compete at Olympics despite ruptured ACL
-
Germany acquires power grid stake from Dutch operator

-
 Finland building icebreakers for US amid Arctic tensions
Finland building icebreakers for US amid Arctic tensions
-
Petro extradites drug lord hours before White House visit

-
 Disney names theme parks boss chief Josh D'Amaro as next CEO
Disney names theme parks boss chief Josh D'Amaro as next CEO
-
Macron says work under way to resume contact with Putin

-
 Prosecutors to request bans from office in Le Pen appeal trial
Prosecutors to request bans from office in Le Pen appeal trial
-
Tearful Gazans finally reunite after limited Rafah reopening

-
 Iran president confirms talks with US after Trump's threats
Iran president confirms talks with US after Trump's threats
-
Spanish skater allowed to use Minions music at Olympics

-
 Fire 'under control' at bazaar in western Tehran
Fire 'under control' at bazaar in western Tehran
-
Howe trusts Tonali will not follow Isak lead out of Newcastle

-
 Vonn to provide injury update as Milan-Cortina Olympics near
Vonn to provide injury update as Milan-Cortina Olympics near
-
France summons Musk for 'voluntary interview', raids X offices

-
 US judge to hear request for 'immediate takedown' of Epstein files
US judge to hear request for 'immediate takedown' of Epstein files
-
Russia resumes large-scale strikes on Ukraine in glacial temperatures

-
 Fit-again France captain Dupont partners Jalibert against Ireland
Fit-again France captain Dupont partners Jalibert against Ireland
-
French summons Musk for 'voluntary interview' as authorities raid X offices

-
 IOC chief Coventry calls for focus on sport, not politics
IOC chief Coventry calls for focus on sport, not politics
-
McNeil's partner hits out at 'brutal' football industry after Palace move collapses

-
 Proud moment as Prendergast brothers picked to start for Ireland
Proud moment as Prendergast brothers picked to start for Ireland
-
Germany has highest share of older workers in EU

-
 Teen swims four hours to save family lost at sea off Australia
Teen swims four hours to save family lost at sea off Australia
-
Ethiopia denies Trump claim mega-dam was financed by US

-
 Russia resumes strikes on freezing Ukrainian capital ahead of talks
Russia resumes strikes on freezing Ukrainian capital ahead of talks
-
Malaysian court acquits French man on drug charges

-
 Switch 2 sales boost Nintendo results but chip shortage looms
Switch 2 sales boost Nintendo results but chip shortage looms
-
From rations to G20's doorstep: Poland savours economic 'miracle'

-
 Russia resumes strikes on freezing Ukrainian capital
Russia resumes strikes on freezing Ukrainian capital
-
'Way too far': Latino Trump voters shocked by Minneapolis crackdown

-
 England and Brook seek redemption at T20 World Cup
England and Brook seek redemption at T20 World Cup
-
Coach Gambhir under pressure as India aim for back-to-back T20 triumphs

-
 'Helmets off': NFL stars open up as Super Bowl circus begins
'Helmets off': NFL stars open up as Super Bowl circus begins
-
Japan coach Jones says 'fair' World Cup schedule helps small teams

-
 Do not write Ireland off as a rugby force, says ex-prop Ross
Do not write Ireland off as a rugby force, says ex-prop Ross
-
Winter Olympics 2026: AFP guide to Alpine Skiing races

-
 Winter Olympics to showcase Italian venues and global tensions
Winter Olympics to showcase Italian venues and global tensions
-
Buoyant England eager to end Franco-Irish grip on Six Nations


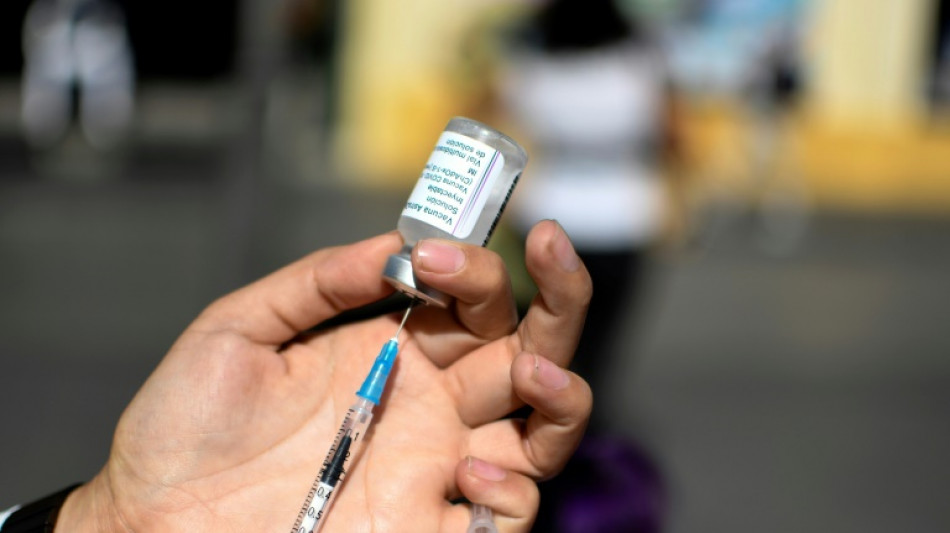
EU drug watchdog approves AstraZeneca Covid prevention jab
The EU's drug watchdog on Thursday recommended for approval AstraZeneca's Covid-19 prevention cocktail, which can be used for patients with immune system problems or severe reactions to other coronavirus vaccines.
The European Medicines Agency's human medicines committee "has recommended granting a marketing authorisation for Evusheld, developed by AstraZeneca for the prevention of Covid-19 in adults and adolescents from 12 years of age," the Amsterdam-based EMA said in a statement.
Evusheld consists of two monoclonal antibodies tixagevimab and cilgavimab -- proteins designed to attack the spike protein of the Sars-CoV-2 virus which causes Covid-19 -- at two different sites, the EMA said.
It said data from a test on 5,000 people who were given two jabs, showed it reduced the risk of Covid-19 infection by 77 percent and protection lasted for at least six months.
The study was done on adults who had never had Covid-19 and had never received a vaccine or other preventative treatment, the EMA said.
"The safety profile of Evusheld was favourable and side effects were generally mild, with a small number of people reporting reactions at the injection site or hypersensitivity," the medicines watchdog added.
But the study was done before the emergence of the infectious Omicron strain of the virus and "laboratory studies show that the Omicron BA.1 variant may be less sensitive to tixagevimab and cilgavimab than the Omicron BA.2 variant," the watchdog said.
The EMA's recommendation will now be forwarded to the European Commission for final approval before distribution to the 27-member bloc.
Evusheld received the US-based FDA's emergency authorisation in December.
M.A.Vaz--PC



