-
 Vonn to provide injury update as Milan-Cortina Olympics near
Vonn to provide injury update as Milan-Cortina Olympics near
-
France summons Musk for 'voluntary interview', raids X offices

-
 US judge to hear request for 'immediate takedown' of Epstein files
US judge to hear request for 'immediate takedown' of Epstein files
-
Russia resumes large-scale strikes on Ukraine in glacial temperatures

-
 Fit-again France captain Dupont partners Jalibert against Ireland
Fit-again France captain Dupont partners Jalibert against Ireland
-
French summons Musk for 'voluntary interview' as authorities raid X offices

-
 IOC chief Coventry calls for focus on sport, not politics
IOC chief Coventry calls for focus on sport, not politics
-
McNeil's partner hits out at 'brutal' football industry after Palace move collapses

-
 Proud moment as Prendergast brothers picked to start for Ireland
Proud moment as Prendergast brothers picked to start for Ireland
-
Germany has highest share of older workers in EU

-
 Teen swims four hours to save family lost at sea off Australia
Teen swims four hours to save family lost at sea off Australia
-
Ethiopia denies Trump claim mega-dam was financed by US

-
 Russia resumes strikes on freezing Ukrainian capital ahead of talks
Russia resumes strikes on freezing Ukrainian capital ahead of talks
-
Malaysian court acquits French man on drug charges

-
 Switch 2 sales boost Nintendo results but chip shortage looms
Switch 2 sales boost Nintendo results but chip shortage looms
-
From rations to G20's doorstep: Poland savours economic 'miracle'

-
 Russia resumes strikes on freezing Ukrainian capital
Russia resumes strikes on freezing Ukrainian capital
-
'Way too far': Latino Trump voters shocked by Minneapolis crackdown

-
 England and Brook seek redemption at T20 World Cup
England and Brook seek redemption at T20 World Cup
-
Coach Gambhir under pressure as India aim for back-to-back T20 triumphs

-
 'Helmets off': NFL stars open up as Super Bowl circus begins
'Helmets off': NFL stars open up as Super Bowl circus begins
-
Japan coach Jones says 'fair' World Cup schedule helps small teams

-
 Do not write Ireland off as a rugby force, says ex-prop Ross
Do not write Ireland off as a rugby force, says ex-prop Ross
-
Winter Olympics 2026: AFP guide to Alpine Skiing races

-
 Winter Olympics to showcase Italian venues and global tensions
Winter Olympics to showcase Italian venues and global tensions
-
Buoyant England eager to end Franco-Irish grip on Six Nations

-
 China to ban hidden car door handles in industry shift
China to ban hidden car door handles in industry shift
-
Sengun leads Rockets past Pacers, Ball leads Hornets fightback

-
 Waymo raises $16 bn to fuel global robotaxi expansion
Waymo raises $16 bn to fuel global robotaxi expansion
-
Netflix to livestream BTS comeback concert in K-pop mega event

-
 Rural India powers global AI models
Rural India powers global AI models
-
Equities, metals, oil rebound after Asia-wide rout

-
 Bencic, Svitolina make history as mothers inside tennis top 10
Bencic, Svitolina make history as mothers inside tennis top 10
-
Italy's spread-out Olympics face transport challenge

-
 Son of Norway crown princess stands trial for multiple rapes
Son of Norway crown princess stands trial for multiple rapes
-
Side hustle: Part-time refs take charge of Super Bowl

-
 Paying for a selfie: Rome starts charging for Trevi Fountain
Paying for a selfie: Rome starts charging for Trevi Fountain
-
Faced with Trump, Pope Leo opts for indirect diplomacy

-
 NFL chief expects Bad Bunny to unite Super Bowl audience
NFL chief expects Bad Bunny to unite Super Bowl audience
-
Australia's Hazlewood to miss start of T20 World Cup

-
 Bill, Hillary Clinton to testify in US House Epstein probe
Bill, Hillary Clinton to testify in US House Epstein probe
-
Cuba confirms 'communications' with US, but says no negotiations yet

-
 From 'watch his ass' to White House talks for Trump and Petro
From 'watch his ass' to White House talks for Trump and Petro
-
Trump says not 'ripping' down Kennedy Center -- much

-
 Sunderland rout 'childish' Burnley
Sunderland rout 'childish' Burnley
-
Musk merges xAI into SpaceX in bid to build space data centers

-
 Former France striker Benzema switches Saudi clubs
Former France striker Benzema switches Saudi clubs
-
Sunderland rout hapless Burnley

-
 Costa Rican president-elect looks to Bukele for help against crime
Costa Rican president-elect looks to Bukele for help against crime
-
Hosts Australia to open Rugby World Cup against Hong Kong


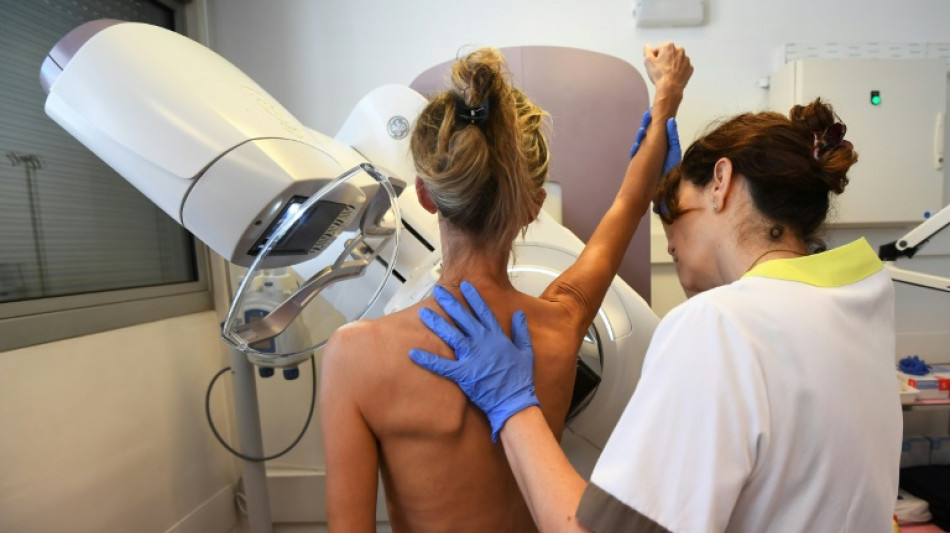
New hope for patients with less common breast cancer
A new treatment nearly halves the risk of disease progression or death from a less common form of breast cancer that hasn't seen major drug advances in over a decade, researchers reported Monday.
Results from the study, presented at the annual meeting of the American Society for Clinical Oncology, are expected to be submitted to regulators and could soon establish a new first-line therapy for people with HER2-positive metastatic breast cancer -- the advanced stage of a form that comprises 15–20 percent of all breast cancer cases.
HER2-positive cancers are fueled by an overactive HER2 gene, which makes too much of a protein called human epidermal growth factor receptor 2 that helps cancer cells grow and spread.
Patients with HER2-positive breast cancer that has spread to other parts of the body live around five years.
"Seeing such a striking improvement was really impressive to us -- we were taking a standard and almost doubling how long patients could have their cancer controlled for," oncologist Sara Tolaney, chief of the breast oncology division at Dana-Farber Cancer Institute, told AFP.
The current standard of care, known as THP, combines chemotherapy with two antibodies that block growth signals from the HER2 protein. The new approach uses a drug called trastuzumab deruxtecan (T-DXd), an antibody attached to a chemotherapy drug.
- 'Smart bomb' -
This "smart bomb" strategy allows the drug to target cancer cells directly. "You can bind to the cancer cell and dump all that chemo right into the cancer cells," explained Tolaney.
"Some people call them smart bombs because they're delivering chemo in a targeted fashion -- which is how I think we're able to really increase efficacy so much."
Common side effects included nausea, diarrhea and a low white blood cell count, with a less common effect involving lung scarring.
T-DXd is already approved as a "second-line" option -- used when first-line treatments stop working. But in the new trial, it was given earlier, paired with another antibody, pertuzumab.
In a global trial led by Tolaney, just under 400 patients were randomly assigned to receive T-DXd in combination with pertuzumab, thought to enhance its effects.
A similar number received the standard THP regimen. A third group, who received T-DXd without pertuzumab, was also enrolled -- but those results haven't yet been reported.
- 44 percent risk reduction -
At a follow-up of 2.5 years, the T-DXd and pertuzumab combination reduced the risk of disease progression or death by 44 percent compared to standard care.
Fifteen percent of patients in the T-DXd group saw their cancer disappear entirely, compared to 8.5 percent in the THP group.
Because this was an interim analysis, the median progression-free survival -- meaning the point at which half the patients had seen their cancer return or worsen -- was 40.7 months with the new treatment, compared to 26.9 months with the standard, and could rise further as more data come in.
Tolaney said the results would be submitted to regulators around the world, including the US Food and Drug Administration, and that future work would focus on optimizing how long patients remain on the treatment, particularly those showing complete remission.
"This represents a new first-line standard treatment option for HER2-positive metastatic breast cancer," said Dr. Rebecca Dent, a breast cancer specialist at the National Cancer Center Singapore who was not involved in the study
G.Machado--PC



