
-
 Olympic big air champion Su survives scare
Olympic big air champion Su survives scare
-
89 kidnapped Nigerian Christians released

-
 Cuba willing to talk to US, 'without pressure'
Cuba willing to talk to US, 'without pressure'
-
Famine spreading in Sudan's Darfur, UN-backed experts warn

-
 2026 Winter Olympics flame arrives in Milan
2026 Winter Olympics flame arrives in Milan
-
Congo-Brazzaville's veteran president declares re-election run

-
 Olympic snowboard star Chloe Kim proud to represent 'diverse' USA
Olympic snowboard star Chloe Kim proud to represent 'diverse' USA
-
Iran filmmaker Panahi fears Iranians' interests will be 'sacrificed' in US talks

-
 Leicester at risk of relegation after six-point deduction
Leicester at risk of relegation after six-point deduction
-
Deadly storm sparks floods in Spain, raises calls to postpone Portugal vote

-
 Trump urges new nuclear treaty after Russia agreement ends
Trump urges new nuclear treaty after Russia agreement ends
-
'Burned in their houses': Nigerians recount horror of massacre

-
 Carney scraps Canada EV sales mandate, affirms auto sector's future is electric
Carney scraps Canada EV sales mandate, affirms auto sector's future is electric
-
Emotional reunions, dashed hopes as Ukraine soldiers released

-
 Bad Bunny promises to bring Puerto Rican culture to Super Bowl
Bad Bunny promises to bring Puerto Rican culture to Super Bowl
-
Venezuela amnesty bill excludes gross rights abuses under Chavez, Maduro

-
 Lower pollution during Covid boosted methane: study
Lower pollution during Covid boosted methane: study
-
Doping chiefs vow to look into Olympic ski jumping 'penis injection' claims

-
 England's Feyi-Waboso in injury scare ahead of Six Nations opener
England's Feyi-Waboso in injury scare ahead of Six Nations opener
-
EU defends Spain after Telegram founder criticism

-
 Novo Nordisk vows legal action to protect Wegovy pill
Novo Nordisk vows legal action to protect Wegovy pill
-
Swiss rivalry is fun -- until Games start, says Odermatt

-
 Canadian snowboarder McMorris eyes slopestyle after crash at Olympics
Canadian snowboarder McMorris eyes slopestyle after crash at Olympics
-
Deadly storm sparks floods in Spain, disrupts Portugal vote

-
 Ukrainian flag bearer proud to show his country is still standing
Ukrainian flag bearer proud to show his country is still standing
-
Carney scraps Canada EV sales mandate

-
 Morocco says evacuated 140,000 people due to severe weather
Morocco says evacuated 140,000 people due to severe weather
-
Spurs boss Frank says Romero outburst 'dealt with internally'

-
 Giannis suitors make deals as NBA trade deadline nears
Giannis suitors make deals as NBA trade deadline nears
-
Carrick stresses significance of Munich air disaster to Man Utd history

-
 Record January window for transfers despite drop in spending
Record January window for transfers despite drop in spending
-
'Burned inside their houses': Nigerians recount horror of massacre

-
 Iran, US prepare for Oman talks after deadly protest crackdown
Iran, US prepare for Oman talks after deadly protest crackdown
-
Winter Olympics opening ceremony nears as virus disrupts ice hockey

-
 Mining giant Rio Tinto abandons Glencore merger bid
Mining giant Rio Tinto abandons Glencore merger bid
-
Davos forum opens probe into CEO Brende's Epstein links

-
 ECB warns of stronger euro impact, holds rates
ECB warns of stronger euro impact, holds rates
-
Famine spreading in Sudan's Darfur, warn UN-backed experts

-
 Lights back on in eastern Cuba after widespread blackout
Lights back on in eastern Cuba after widespread blackout
-
Russia, US agree to resume military contacts at Ukraine talks

-
 Greece aims to cut queues at ancient sites with new portal
Greece aims to cut queues at ancient sites with new portal
-
No time frame to get Palmer in 'perfect' shape - Rosenior

-
 Stocks fall as tech valuation fears stoke volatility
Stocks fall as tech valuation fears stoke volatility
-
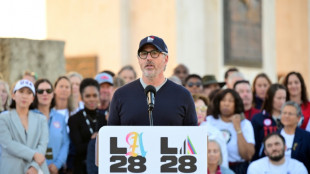
US Olympic body backs LA28 leadership amid Wasserman scandal
-
 Gnabry extends Bayern Munich deal until 2028
Gnabry extends Bayern Munich deal until 2028
-
England captain Stokes suffers facial injury after being hit by ball

-
 Italy captain Lamaro amongst trio set for 50th caps against Scotland
Italy captain Lamaro amongst trio set for 50th caps against Scotland
-
Piastri plays down McLaren rivalry with champion Norris

-
 ECB holds interest rates as strong euro causes jitters
ECB holds interest rates as strong euro causes jitters
-
EU close to sealing trade deal with Australia


Florence Announces AI-Assisted Workflows to Transform Clinical Trials
ATLANTA, GA / ACCESS Newswire / September 2, 2025 / Ahead of its premier life science event advancing Clinical Trial technology and innovation, Research Revolution, Florence Healthcare today announced its upcoming AI-centric workflows aimed at transforming clinical trials conducted across the study life-cycle. These solutions will significantly accelerate and enhance site identification, feasibility, start up, study conduct and remote monitoring for both sites and sponsors.
Built in collaboration with top sponsors, CRO partners, and hundreds of research sites; these new Human-in-loop (HITL) AI capabilities are built to supercharge clinical trial professionals as they tackle some of the most persistent challenges in the industry:
Limited Visibility Into Site Performance: Sponsors struggle to identify the right research sites, relying on disparate and stagnant data sources that provide limited visibility into true site capacity and performance.
Inefficient Study Start-Up Processes: Timelines are bogged down by manual and fragmented workflows and communication across stakeholders. Meaning critical data and documents are trapped in email chains, portals, and spreadsheets- causing version control issues, compliance risks, and inefficiencies.
Fragmented Oversight & Monitoring: Fragmented data and documents means that sponsor oversight is limited. Disparate systems prevent monitors from having real-time visibility into trial progress. As a consequence, costly physical site visits are still needed.
AI Assisted workflows will be available to Sponsors, CROs and Research Sites in October 2025
Florence Healthcare will introduce modules to directly address the challenges listed above. These will include:
Site Identification & Feasibility: Utilizing these new workflows, Sponsors will be able to quickly search across site profiles according to therapeutic area, geography, and real-time performance criteria to pick the right sites for study startup. From there they can deliver protocol-specific feasibility surveys, focusing on questions that are informative rather than repetitive. Sites will then be able to leverage an AI-driven workflow to accelerate survey completion with context-specific responses highlighting Site qualifications for the specific study.
Study Start-Up: New AI-driven contract negotiations will accelerate time to startup, in compliance with Site and Sponsor guidelines for contracts. In addition, a fully automated document exchange with Site eISF will provide the flexibility to tailor documents to specific Site networks or regions. The enhanced AI workflows will also simplify document management across startup and trial conduct, reducing administrative work for sites and sponsors.
Remote Monitoring: Remote Monitoring will also receive an AI upgrade. New reports providing deeper insights into Study progress across Sites or at specific sites will be available, allowing for quick intervention and remediation to keep trials on track, and obviating the need for physical site visits.
These AI-centric product workflows will enter limited availability in October 2025 for early adopters. These workflows are built on Florence's validated and GxP-compliant infrastructure-trusted by over 37,000 research sites globally. They include full audit traceability, 21 CFR Part 11 support, and interoperability APIs for CTMS, eTMF, and identity systems.
"We're not just automating the trial conduct for speed," said Shankar Jagannathan, COO at Florence. "We're creating a shared, AI-augmented experience between sponsors and sites to reduce manual work and increasing capacity, while minimizing risk -one that's more intelligent, scalable, and efficient."
About Florence Healthcare
Florence is a purpose-built platform that connects sponsors and sites to accelerate clinical trials, improve operational capacity, and reduce risk. Designed for scale, Florence streamlines workflows, enhances collaboration, and delivers real-time visibility across studies-empowering research teams to move faster, stay inspection-ready, and increase trial throughput with fewer resources.
For more information, visit www.florencehc.com
Press Contact:
Lautel Okhio
Communications, Florence
[email protected]
SOURCE: Florence Healthcare
View the original press release on ACCESS Newswire
E.Paulino--PC
